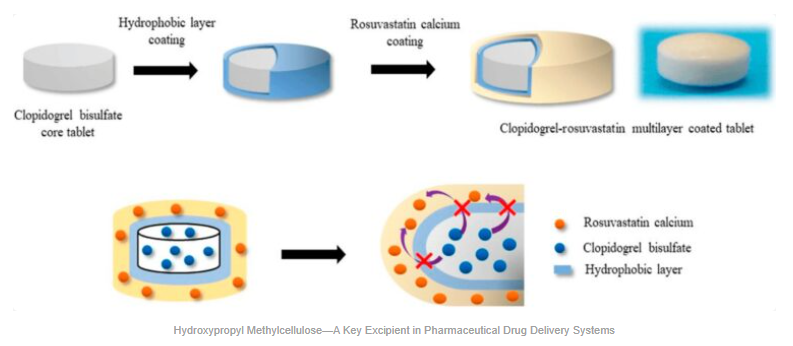
Hydroxypropyl methylcellulose—a key excipient in pharmaceutical drug delivery systems
Hydroxypropyl Methylcellulose (HPMC), also known as hypromellose, is a semi-synthetic polymer derived from cellulose that plays a crucial role as a pharmaceutical excipient. Its unique properties, including water solubility and gel-forming ability, make it indispensable in various drug delivery systems. HPMC is widely used in controlled and sustained release formulations, enabling the gradual release of active ingredients to improve therapeutic efficacy and patient compliance. It also serves as a binder and film-forming agent in tablets and capsules, enhancing their mechanical strength and stability. Beyond oral dosage forms, HPMC’s biocompatibility and viscosity make it suitable for ophthalmic preparations like eye drops. Overall, HPMC’s versatility and safety profile make it a cornerstone excipient in modern pharmaceutical manufacturing.
Comments
No comments posted yet.