Optimizing API load and minimizing tablet weight leveraging an innovative DC Mannitol
*INTRODUCTION
Tablets with stability issues often utilize mannitol, a stable, water-soluble excipient. Direct compression is a preferred method for its simplicity and cost-effectiveness. However, directly compressible mannitol grades tend to cap under high tableting speeds or compression forces. Solutions to capping, such as precompression, reduced production speed, or lower compression force, usually require reducing the API load.
*OBJECTIVES
PEARLITOL® 200 GT is a granulated beta mannitol designed for direct compression, optimized to improve tabletability and reduce capping. Its high powder density enhances tabletability and prevents capping, allowing for increased API load and smaller tablet size for the same dosage. The study used sertraline, a poorly tabletable API, to test these improvements against a reference spray-dried mannitol (PEARLITOL® 200 SD).
*MATERIALS AND METHODS
Materials included granulated mannitol (PEARLITOL® 200 GT), spray-dried mannitol (PEARLITOL® 200 SD), magnesium stearate, and sertraline. Mannitol and sertraline were mixed, with magnesium stearate added subsequently. Powder properties such as bulk density, tapped density, Hausner ratio, flow time, and angle of repose were measured. All tableting trials were done on a high-speed rotary tablet press simulator STYLCAM 200R (MEDELPHARM) using KORSCH XL 400 profile.
*RESULTS
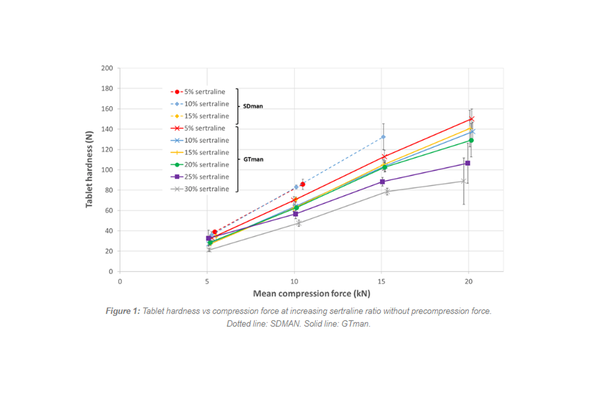
Sertraline powder had poor flow properties. Blends with PEARLITOL® 200 SD were free-flowing up to 15% sertraline. Blends with PEARLITOL® 200 GT were free-flowing up to 25%, allowing tablet production up to 40% sertraline. Without precompression, PEARLITOL® 200 SD exhibited capping at all sertraline ratios, while PEARLITOL® 200 GT did not cap up to 25% sertraline. With precompression, PEARLITOL® 200 GT produced viable tablets up to 40% sertraline, while PEARLITOL® 200 SD capped at 15% sertraline.
*CONCLUSION
Using PEARLITOL® 200 GT for direct compression significantly improves tabletability, allowing for higher API loads or smaller tablets. PEARLITOL® 200 GT enabled up to a 166% increase in API load or a 62% reduction in tablet weight compared to PEARLITOL® 200 SD.
Comments
No comments posted yet.