Pharmaceutical granulation: enhancing drug manufacturing efficiency
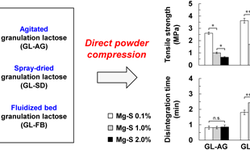
Granulation is a vital process in pharmaceutical manufacturing, where fine powder particles are combined into larger, free-flowing granules. This process is crucial for enhancing the physical properties of drug formulations, improving powder flow, minimizing dust, and ensuring uniform content in solid dosage forms such as tablets and capsules. The pharmaceutical industry uses various granulation methods, including wet and dry techniques, each with distinct advantages. This infographic highlights key wet granulation methods and their benefits. Download now to discover how choosing the right granulation technique can optimize production, boost product quality, and increase overall manufacturing efficiency.

Comments
No comments posted yet.