Validation 4.0 for the pharmaceutical industry
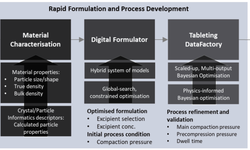
The pharmaceutical industry is under pressure to develop new therapies faster while maintaining high quality and a robust supply chain. Validation 4.0 is an emerging approach that transforms traditional validation practices into a digital, data‑driven, continuous strategy that supports rapid innovation without sacrificing regulatory compliance. It integrates technologies such as artificial intelligence (AI), the Internet of Things (IoT), cloud computing, and digital twins with established frameworks like Quality by Design (QbD) and quality risk management (QRM) to monitor and control manufacturing processes in real time.
Rather than relying on conventional project‑based methods and fixed validation runs, Validation 4.0 emphasizes ongoing evidence generation and digital control, which helps pharmaceutical organizations adapt more quickly to new therapies, continuous production methods, and complex manufacturing environments. For tablet and solid dosage manufacturing, this means increased process visibility, faster issue detection, and stronger control strategies throughout the product lifecycle, enabling manufacturers to accelerate development and improve outcomes in a highly regulated market.

Comments
No comments posted yet.