The Companies
Accord Healthcare is involved in the distribution and manufacturing of pharmaceutical products to over 35 European countries.
It has one of the largest market footprints of any European generics and biosimilar company and is listed as the fastest growing generics and biosimilar company in the world.
Accord Healthcare’s vision is to become a top 5 pan-region generics company by 2021. Their mission is to generate sustainable growth in sales, volume and company value, in the areas of generics, added value medicines, biopharmaceutical and specialty pharmaceuticals, whilst ensuring that their patients get access to high-quality affordable medicines. They are also committed to developing the skills and capabilities of their people.
Headquartered in the UK, the company has an extensive supply chain through its four UK based sites, helping to ensure a consistent supply of life-saving medicines for patients, whilst supporting customers to react quickly to dynamic market conditions.
With a history of over 70 years, I Holland has built and maintained its position as one of the World’s leading suppliers of tablet compression tools to pharmaceutical, nutraceutical and confectionery industries across the globe.
The company has been instrumental in introducing many innovative and unique developments to the industry. Working closely with its customers, I Holland helps to ensure clients operate the best possible tablet manufacturing processes.
The product
One of the products Accord Healthcare manufactures is a 12.5mm Flat Bevelled Edged tablet embossed with central break-line.
Problem 1
Previously, Accord Healthcare used standard Tungsten Carbide dies to compress this product but due to ‘black spots’ appearing on the tablet walls after compression, the usage of Tungsten Carbide dies was suspended in 2008 and over the years either premium steel dies or powder metallurgy dies were used to compress the product.
At the time it was believed the problem was being caused through the added resistance during tablet ejection, or through a reaction with the Tungsten Carbide material itself.
Tungsten Carbide dies have been adopted as an industry standard for dies when the highest level of wear resistance is required. It features a high compressive strength with an extremely high wear resistance. This helps to reduce die bore wear and ringing and lasts longer than conventional die steels.
Although it is a tooling material commonly used within the pharmaceutical industry, normal Tungsten Carbide uses Cobalt as a binding material, this can have reactive properties with acids and certain materials found within formulations and can cause corrosion resulting in black spots on the tablets.
I Holland has developed a Tungsten Carbide grade that uses a special binder in replacement of cobalt, the binder has exceptional corrosion resistance meaning it makes an excellent option if both corrosion protection and resistance to abrasive wear are required.
Problem 2
During manufacture, another problem experienced by Accord Healthcare was the capping and delamination of the tablets. Capping is when the top of the tablet separates horizontally when ejected from the press. Delamination is when the tablet splits apart along the layers.
There can be several possible causes for this outcome during tablet production:
- Burred/clawed punch tips
- Too many fines in the granulation
- Weak granule
- Worn Die bores (Die ringing)
- Compression occurring too low in die boreOver/under pressure being applied Air Entrapment in the tablet body.
The Investigation
In order to find resolutions to the problems being experienced during the manufacture of this product I Holland undertook a trial using their non-marking HPG-TC (Tungsten
Carbide) dies.
Sample dies from several sets (ranging from 2007-2018) were supplied to I Holland for analysis. The first step was to use dimensional analysis of the dies through the ‘Approve’ system. This works by using a combination of technologies/measuring equipment to accurately determine the dimensions of punches and dies to micron level. The Approve
system’s quickset fixtures are optimised to be fast and repeatable, measuring tooling in all critical dimensions. This allows the data to be transferred to software systems such as I Holland’s Tool Management system (IH-TMS) which creates inspection reports.
The reports were able to confirm that the clearance between the punch tip outer diameters and die bores were well within specification. This eliminated this as a cause of the delamination.
The next process was to use surface finish analysis. A form Talysurf was used to measure the surface finish of the die bores to ensure they were manufactured to I Holland Eurostandard specifications. I Holland’s Eurostandard document advises manufacturers on tablet tooling terminology, procurement, configuration and includes the latest technical specification information.
It was found the die bore surface finish was in specification for all the measured dies, so a ‘roughening’ of the die bore was ruled out as a cause of the issues.
Finally, analysis of the sample tooling took place using iNSPECT 2.0. Punches and dies should be visually inspected to establish if the tablet production process is running well and identify whether tooling maintenance is required. This assessment should also be carried out under magnification for any signs of damage, wear or corrosion, and to validate the cleaning process.
Carrying out an assessment will establish if the production process is working as it should, giving clues to problems with the tablet press or tooling itself, and if any tooling maintenance is required. Typical equipment that should be used for the inspection includes high magnification lenses and microscopes. I Holland’s iNSPECT 2.0, visual camera system was used in this trial. Due to the high mirror finish of tablet tooling, it has been specially developed for the close inspection of punch tips and tablets. Any system that is used should be precise and give a clear high quality image that can be used to identify any problems.
The Trial
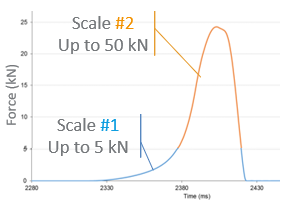
A compression trial was undertaken on-site at Accord Healthcare using I Holland’s newly developed special grade of Tungsten Carbide. The trial included manufacturing 4 batches of tablets with a production time of around 5 hours and 1.6 million tablets per batch. Inspection of 5 specific dies took place after each batch to monitor any change in die bore appearance, for example, signs of wear, and photographs were taken for record and stored to enable comparisons to be made during the trial.
Solution 1
After the trial using the new Tungsten Carbide dies , no black marks were found on the tablet side walls.
Analysis on-site showed that the dies and punch tips had sufficient clearance when fitted to the press. The temperature influence (27.3oC at full speed compression) was checked during the site visit and determined not to contribute to the cause of the capping and delamination or black marks on the tablets (i.e. there was no heat created through friction that could have melted the granule). The press produced 240,000 tablets per hour with a batch size of 1.6 million tablets.
Solution 2
Following extensive analysis of the supplied dies, I Holland recognised that the main cause for the capping and delamination was through die bore ringing. The rings can either be in the form of one ring which is caused by an abrasive granule, or by two rings which indicates tip deflection. ‘Die bore ringing’, takes place in the die bore where the tablet is compacted. The wear appears as a double ring due the upper and lower punch tips flexing and bending within the clearance between the tips and the die bores during full compaction. The tablet when formed within these rings can have issues when being ejected from the die causing capping and delamination of the tablet.
Analysis of the rings on the old dies revealed that the depth of the die bore ringing varied between 3 and 17 microns, the deeper depth being with Premium HPG steel dies as opposed to the HPG Powder Metallurgy Dies. These use a specialist powder metallurgy grade steel with uniform carbide distribution and small carbide size giving extremely high wear resistance over and above that of premium steel, but it is less wear resistant than Tungsten carbide.
Analysis has shown that the life expectancy of the dies has always been inadequate due to the compromise of material selection required to overcome the black marks. This has necessitated in sets of dies being discarded due to capping and delamination problems with the tablets.
Conclusion
The results of this trial established that through using material specifically developed for wear, in this case I Holland’s specially developed Tungsten Carbide material, problems like black marks caused through corrosion and capping issues are eliminated from production. In addition, the specially developed Tungsten Carbide dies have very high wear resistance therefore allowing Accord healthcare to use the most wear resistant material available minimising issues with production.
To clearly define why any production problems like black spots or capping are taking place, it is important to consult with a trusted tooling expert. With extensive technical know-how and many years of analysis the correct tooling specifications can be implemented resulting in improved tablet production.