Tablet formulation development
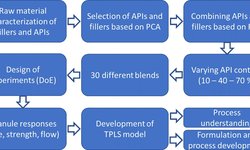
Understand powder behavior to develop robust formulations. API characterization, excipient selection, and manufacturing process determination are essential in every tablet development to achieve a robust formulation.
Study formulation blends and manufacturing processes following USP <1062> guidelines with our pharmacists to meet tablet Critical Quality Attributes (CQAs) such as dissolution, disintegration, or tensile strength. As an example, assess the impact of dry granulation process on flowability and re-compactibility with a minimal amount of material.
Design the process to maximize production output, control cost, and ensure superior quality. Compaction simulators are essential to predict potential tablet defects and solve them during the development stage. For example, sticking can be related to temperature or tooling surface which can be evaluated with our formulators prior to scale-up.
Our pharmacists and formulation experts provide insights based on experiments and data to define the best combination of excipients and processes to target optimal quality attributes.
Articles you may like
Related products

Looking for additional information?
Let us help you