Right from the start: designing advanceable formulations and processes at phase I
The text discusses the importance of designing drug formulations and processes that are advanceable from the early stages of development, emphasizing safety, efficacy, manufacturability, and commercial viability. It highlights the benefits of accelerating drug development timelines, reducing costs, and increasing revenue through early emphasis on commercial readiness. The focus has shifted towards assessing drug efficacy in phase I rather than waiting for phase II studies, with an emphasis on flexible formulations and processes to support rapid advancement through clinical trials and commercialization.
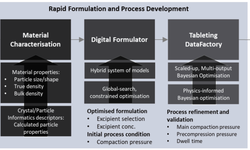
Serán Bioscience exemplifies this approach by starting discussions with clients before phase I, tailoring development plans, and employing material-sparing approaches to maximize speed and flexibility while ensuring scalability and compliance with regulatory standards. They emphasize the importance of integrating material science principles into drug development to support early-stage studies and offer flexible solutions for oral solid dosage forms.
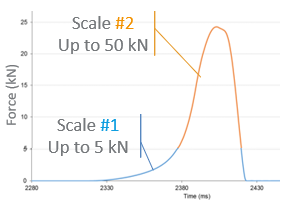
Tablets can be quickly developed using minimal powder quantities by assessing material properties during fomrulation screening and identifying scale factors. The selection of excipients, tablet structure, and processing methods can impact factors like compressiblity, tabletability, and compactibility (CTC). Tools like the STYL'One Nano compaction simulator (MEDELPHARM/KORSCH) allow for the evaluation of material properties using only one or two tablets, providing crucial insights to streamline the scaling process efficiently.

Comments
No comments posted yet.