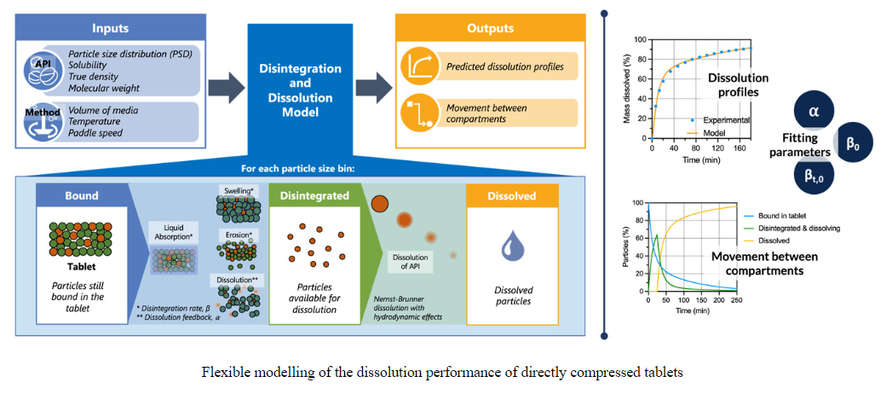
Flexible modelling of the dissolution performance of directly compressed tablets
This study proposes a compartmental disintegration and dissolution model to predict and evaluate the dissolution behavior of directly compressed tablets. The model consists of three compartments (Bound, Disintegrated, and Dissolved) to represent the state of each active pharmaceutical ingredient (API) particle. Tablet disintegration is characterized by three fitting parameters: 𝛽0 and 𝛽𝑡,0, which describe the initial disintegration rate and its rate of change, respectively, and a third parameter, 𝛼, which accounts for the effect of the dissolved drug volume on the disintegration process. As the tablet disintegrates, particles become available for dissolution.
The dissolution rate is calculated using the Nernst-Brunner equation, incorporating hydrodynamic effects within the vessel of a USP II (paddle) apparatus. The model utilizes the raw material properties of the API (solubility, particle size distribution, and true density), making it suitable for early development stages when the amount of drug substance may be limited. Moreover, the strong correlations between the fitting parameters and tablet porosity suggest the potential to separate manufacturing effects, enabling the model to be used as part of a real-time release testing strategy for a continuous direct compression process.
Comments
No comments posted yet.