Formulation forum - Orally disintegrating tablets
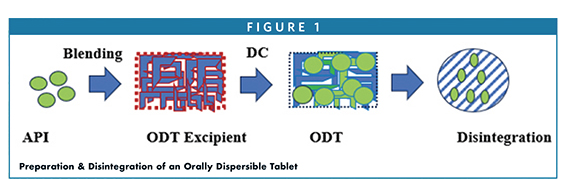
Orally disintegrating tablets (ODTs) are designed to rapidly break apart in the mouth — typically within 30 seconds or less — without the need for water, making them especially useful for pediatric, geriatric, and dysphagic patients. The solid dosage form aims to improve dissolution and onset of action, offering advantages over conventional tablets, including enhanced patient compliance, faster absorption, accurate dosing, and simplified packaging.Drug Development and Delivery
A key driver of ODT performance is the choice of excipient systems that combine low density, high porosity, and rapid hydration with good mouthfeel and taste masking. Many co‑processed excipients (e.g., Ludiflash®, Pearlitol Flash, Prosolv® ODT G2, F‑Melt®, Pharmaburst® 500, StarLac® ODT, and Granfiller‑D™) are based on mannitol plus disintegrants like crospovidone to achieve rapid disintegration while maintaining tablet hardness and friability. Comparative studies show that disintegration times and performance can vary depending on excipient composition and hydration rates.Drug Development and Delivery
Manufacturing of ODTs often uses direct compression for efficiency, though technologies like spray drying, extrusion, lyophilization, and molding are also applied when needed. Because ODTs are sensitive to humidity and physical stress, appropriate storage and packaging — usually moisture‑protective environments — are critical to retain rapid disintegration behavior. Finally, the article notes that ODTs are increasingly part of life‑cycle management strategies and are approved for a wide range of therapeutic indications, reflecting their growing importance in patient‑centric drug delivery.
Comments
No comments posted yet.














