From technology selection to final drug product: material sparing approaches for asd development
Compounds with low solubility and poor bioavailability present ongoing challenges in pharmaceutical development. This webinar explores Lonza's ‘right first time’ strategy, which employs material-efficient and time-saving techniques to accelerate the transition of molecules from development to commercialization.
Key topics include:
- Feasibility screening to identify the best manufacturing method between spray drying (SD) and hot melt extrusion (HME).
- Lonza’s innovative methodology for screening HMEs, particularly at a milligram scale, aimed at accurately representing the extrusion process.
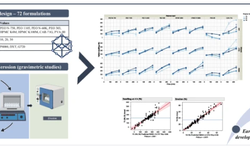
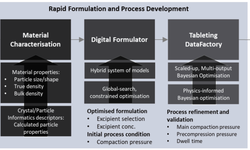
- Advancements in amorphous solid dispersion (ASD) development, from drug product intermediates to final products, with a focus on material-efficient tools for formulation selection and scale-up.
The second part of the webinar will cover effective practices in ASD-based tablet feasibility and demonstration batches. This section will examine:
- The critical differences between spray drying and hot melt extrusion through feasibility screening.
- Material-efficient applications of a compaction simulator to guide tablet development.
- The importance of material-saving techniques in reducing time and costs during the scaling process.

Comments
No comments posted yet.