High-loaded dosage forms
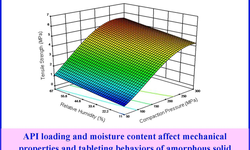
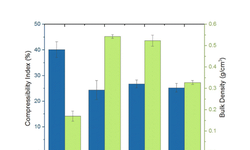
The article "High-Loaded Dosage Forms: Novel Platform Expands Dispersion Utility" presents advancements in developing high drug-loaded amorphous solid dispersions (ASDs) to improve the bioavailability of poorly soluble compounds. Traditional ASD formulations often face challenges such as limited drug loading and physical instability. To overcome these limitations, a novel platform has been developed that combines two different polymers—one inside and one outside the ASD—to maximize drug loading, physical stability, and bioperformance. This high-loaded dosage form (HLDF) approach offers several benefits: it allows higher drug concentrations within the dosage form, improves physical stability by reducing the risk of crystallization, and reduces tablet mass by approximately 40% compared to conventional formulations, potentially enhancing patient compliance. Overall, this platform expands the utility of ASDs by enabling higher drug loadings while maintaining stability and manufacturability, providing a promising strategy for developing oral solid dosage forms with improved bioavailability.
Comments
No comments posted yet.