Scientific papers
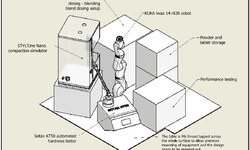
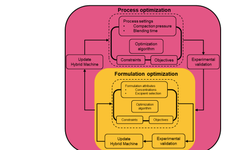
Platform II employs artificial intelligence (AI) to establish an autonomous workflow for the manufacturing and testing of drug products. It achieves this by identifying critical material attributes (CMAs) and their corresponding critical process parameters (CPPs), leading to the attainment of specific critical quality attributes (CQAs). Our objective is to create and leverage a database comprising hundreds of historical and new experiments. This approach aims to mitigate risks and expedite drug product development, concurrently reducing the number of experiments, development time, and material usage by 30%.
Comments
No comments posted yet.
Add a comment