Scientific papers
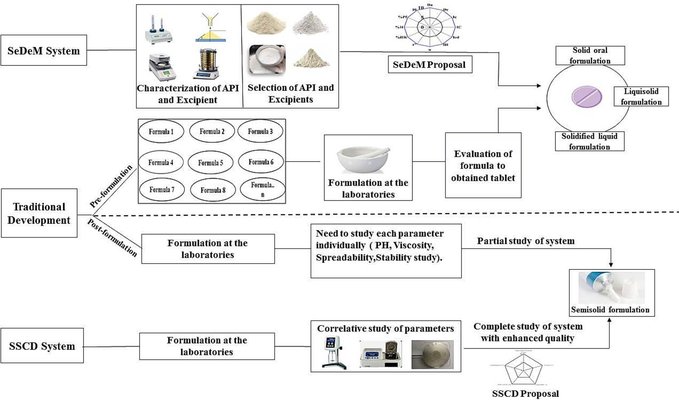
The Sediment Delivery Model (SeDeM) involves the experimental analysis and quantitative evaluation of parameters that characterize powdered substances, offering crucial insights into the material's suitability for the direct compression (DC) of tablets. This approach incorporates mathematical modeling and employs a Semisolid Control Diagram, often implemented through software like iTCM. The SeDeM diagram expert system (DES) evaluates the suitability of both excipients and active ingredients for DC, calculating the API-to-excipient ratio. While DC is known for its time-saving and efficient process, certain excipients may have suboptimal flow properties. Consequently, there was a need for a novel system to streamline the optimization of direct compression tablets, reducing the number of experiments and time required.
The SeDeM DES operates on the principles of quality by design (QbD), as outlined in ICH Q8, assessing critical quality attributes that impact the quality of the finished product. This review predominantly focuses on various dosage forms, encompassing solid, semisolid, liquisolid, and solidified liquid dosage forms. These techniques characterize all substances using 12 parameters, resulting in a 12-sided regular polygon. However, the number of parameters may vary based on the specific requirements of a particular dosage form, such as 15 parameters for an orodispersible tablet and 5 parameters for a semisolid dosage form. The rationale behind establishing limits for these indexes is thoroughly justified.
Comments
No comments posted yet.
Add a comment