Scientific papers
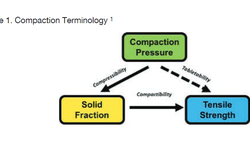
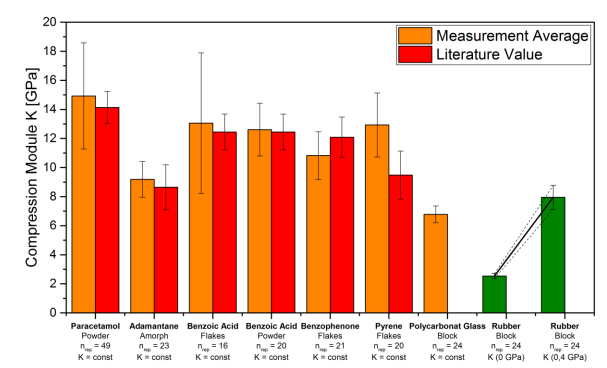
The characterization and development of a tableting process commonly involve investigating compressibility (porosity vs compression stress), tabletability (tensile strength vs compression stress), and compactibility (tensile strength vs porosity), as outlined in USP<1062>. In addition to these out-of-die characteristics, the in-die process can be analyzed using an instrumented tablet press equipped with a high-resolution force–distance measuring system. This approach provides specific energy values, elastic recovery, and in-die compressibility data. The porosity decrease with increasing compression stress is often analyzed through the well-known Heckel plot. Notably, when analyzing the in-die compressibility of materials with low bulk modulus, apparent porosity values below zero are frequently observed at elevated compression stress. However, such occurrences are seldom discussed in the literature. Pragmatic approaches to address these observations include disregarding high compression stress data, adjusting the volume of the compact, or assuming errors in the "true" density values used, often measured by gas-pycnometry.
Comments
No comments posted yet.
Add a comment