A QbD guided development of a Twin-Screw Granulation process
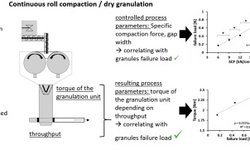
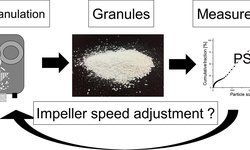
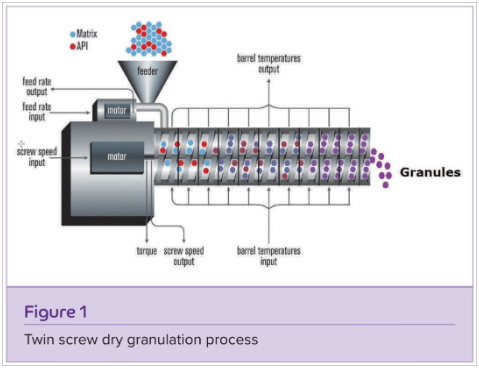
The text discusses dry granulation techniques in the pharmaceutical industry, focusing on roller compaction and slugging, highlighting their limitations such as inferior tablet strength and reduced yield due to fine particulate formation. It introduces a solution to these issues through the use of a twin-screw extruder, explaining its operation and benefits. The process involves inducing interparticular bonding through pressure and various interactions, leading to improved mechanical strength. A Quality Target Product Profile (QTPP) is defined for controlled-release tablets, and a Design of Experiments (DoE) approach is used to optimize factors such as screw speed, feed rate, and formulation. The results indicate the significant effects of these factors on granule properties such as size, dissolution, and flow characteristics. The study demonstrates the successful application of Quality by Design (QbD) principles in developing sustained-release formulations using twin-screw extrusion, offering insights into its potential in pharmaceutical manufacturing.
Comments
No comments posted yet.