Formulation development and evaluation of brivaracetam extended- release tablets by QbD approach
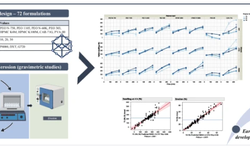
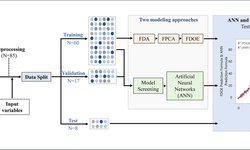
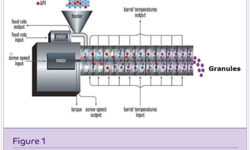
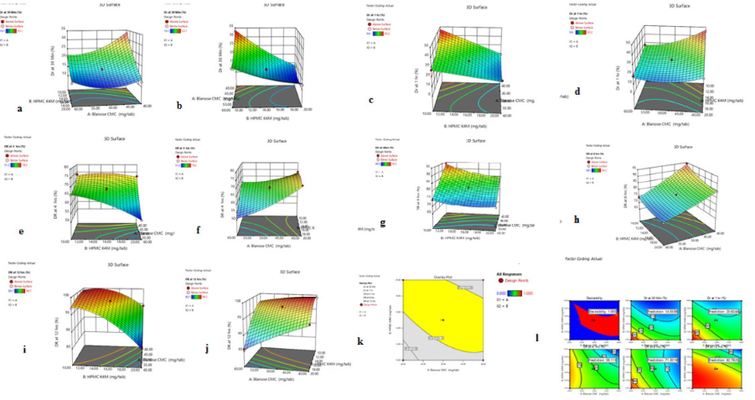
This research article discusses the development and evaluation of extended-release tablets of brivaracetam (BRV), an antiepileptic drug, using a Quality by Design (QbD) approach. Due to BRV's short half-life, it typically requires twice-daily dosing, which can affect patient adherence. The study aims to create a once-daily extended-release formulation to improve compliance. The QbD method involves systematic risk management and quality assurance, supported by regulatory bodies. The research includes selecting optimal polymers, precise formulation processes, and thorough testing to ensure consistent drug release and quality. The identified formulation, BRERT-17, shows promise for better epilepsy management and patient adherence
Comments
No comments posted yet.