Optimal formulation development
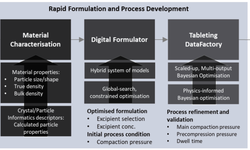
Formulation development is a crucial aspect of the drug development process, involving activities such as developability assessment, buffer optimization, dosage form studies, and compatibility testing. Early investment in formulation development offers significant benefits throughout the drug pipeline by identifying potential risks related to a drug candidate’s properties, including potency, safety, and manufacturability. This early assessment allows for modifications to align with the target product profile (TPP), reducing risks and costs in later stages. The webinar explores how integrating formulation development with drug development and de-risking strategies can optimize drug candidate selection and formulation processes for success.

Comments
No comments posted yet.