Optimizing pediatric product development for commercialization success
Medication adherence in pediatric patients is a growing challenge due to the complexities of their changing physiology and pharmacokinetics at different stages of development, from neonates to adolescents. These variations create significant obstacles in designing and developing formulations tailored to pediatric needs.
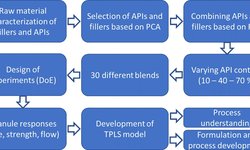
The main hurdles include achieving precise and flexible dosing, improving ease of swallowing, and ensuring palatability. Multiparticulate-based formulations, such as granules, beads, pellets, and minitabs, have emerged as one of the most effective solutions. These systems offer dose flexibility, accuracy, and ease of swallowing and can be delivered in formats like orally disintegrating tablets (ODTs), food sprinkles, reconstitutable powders, or suspensions.
This presentation highlights the importance of the pediatric population, discusses regulatory requirements, and examines the challenges in developing pediatric-specific formulations. It also delves into innovative dosing solutions and strategies, with a focus on multiparticulates, to overcome formulation obstacles.
Watch the webinar to unlock insights into optimizing your pediatric development process.
Key Learning Objectives:
- Understand the importance of the pediatric population, regulatory considerations, and pediatric-friendly product development.
- Identify the unique needs of pediatric patients and the critical role of age-appropriate dosing solutions in improving medication acceptance and adherence.
- Explore the versatility of multiparticulates as a platform for developing child-friendly formulations.

Comments
No comments posted yet.