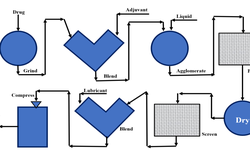
Oral solid dosage formulation and process development
A recent article by Pharmaceutical Online details a strategic, step-by-step approach to the formulation and process development of a preclinical oral solid drug product. The study highlights how to progress efficiently from early-stage API characterization to commercial-scale manufacturing.
Key takeaways include:
- Preclinical development: Emphasis on understanding API properties (e.g., polymorphs, salts) and selecting appropriate excipients for animal and human studies.
- Clinical phases: Liquid formulations are preferred in early human trials, while later phases shift to solid dosage forms—requiring tailored manufacturing strategies.
- Commercialization: Focus on scalable, quality-by-design (QbD) processes that ensure long-term stability and compliance with regulatory standards (e.g., QTPP, SUPAC).
The case study underscores the importance of scientific rigor, cross-functional collaboration, and risk-based decision-making throughout drug product development.

Comments
No comments posted yet.