Sharpening-pharmas-data-integrity-with-automation
The article discusses the increasing regulatory focus on data integrity in the pharmaceutical manufacturing industry, emphasizing the need for complete, consistent, and accurate data. This focus is due to the rise of new treatments and the need for regulatory bodies to ensure data quality for each batch or continuous process.
Key points include:
Importance of Data Integrity:
Good manufacturing practices (GMP) must be followed, and documentation must be verifiable.
Non-compliance can lead to fines, product recalls, increased inspections, and reputational damage.
Automation is recognized as a means to ensure data integrity by minimizing errors, optimizing resources, and reducing patient risk.
Automation and Technology:
Facilities need a distributed control system (DCS) for process automation and a manufacturing execution system (MES) for electronic batch records.
Selecting systems that support ALCOA+ guidelines is critical for ensuring data integrity and GMP compliance.
Risks of Poor Data Integrity:
Common issues include poor batch records and sloppy documentation.
Example: An FDA audit found discrepancies in manufacturing steps documentation, highlighting the risk to the company's reputation.
Systemic issues include insecure logins, improper system administration rights, and issues with electronic signatures and validation documentation.
Improving Data Integrity Compliance:
Leading DCS and MES systems ensure data integrity by incorporating ALCOA+ principles.
Benefits include electronic records, electronic signatures, security controls, revision tracking, and change management.
ALCOA+ Standards:
Attributable: Systems should track who performed tasks and the context.
Legible: DCS and MES eliminate paper records, ensuring data legibility and accessibility.
Contemporaneous: Systems should record actions with time and date stamps using network time protocol.
Original: Validity of data must be proven, with secure storage and restricted device participation.
Accurate: Built-in data protection mechanisms, security controls, and asset management packages ensure accuracy.
Complete: DCS and MES solutions help avoid data loss and ensure comprehensive data collection.
Consistent: Enforced validated workflows ensure consistency.
Enduring/Available: Solutions must work with backup and recovery tools for data availability.
Governance and Management:
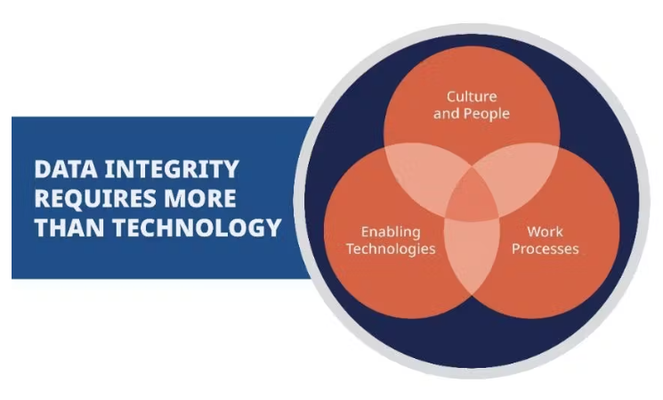
Data integrity requires management processes, procedural and technical controls, and consideration of human factors.
Organizational culture and documented work processes are crucial for ensuring data integrity.
Preparing for Modern Automation:
Future regulations may require more digital plant solutions.
Implementing digital technologies like DCS and MES today helps future-proof investments and drive efficiency and quality.
The text underscores the critical role of automation and proper system selection in maintaining data integrity and compliance in pharmaceutical manufacturing.
Comments
No comments posted yet.