Technical considerations for developing oral solids
Part 1 of the series by Pfizer CentreOne focuses on technical considerations for developing oral solids. The article emphasizes the significance of thorough technical groundwork and understanding excipients during the initial stages of the project.
1. Thorough Technical Groundwork
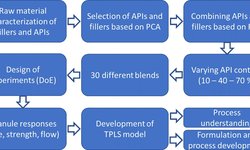
Sandra Conway highlights the importance of a comprehensive understanding of the formulation, material characterization, and process design. This involves assessing the Biopharmaceutics Classification System (BCS) of the API, critical control excipients, dosage form, and process stages. Material characterization and supply chain assessment are crucial in determining material specifications and control strategies, while process design and control require careful consideration of equipment selection, environmental controls, and safety measures.
2. Consideration of Excipients
Dr. Ali Rajabi-Siahboomi and Dr. Alberto Genovesi underscore the importance of excipients in optimizing drug delivery. Considerations include the desired drug release profile, manufacturing processes, potential interactions with the API, film coating selection, and tablet design. Selection and testing of excipients against quality and performance standards are necessary to ensure formulation robustness and stability. Attention is drawn to the impact of excipient properties on manufacturing costs and overall product quality, urging developers to prioritize long-term efficiency over initial cost considerations.
The article emphasizes the significance of collaboration with key suppliers and the use of appropriate technology to develop stable formulations that are easy to scale up and manufacture.

Comments
No comments posted yet.