Winning strategies for oral dosage form development and manufacturing
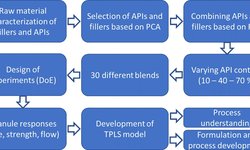
OSD formulation is often complicated by issues such as poor API bioavailability, challenging physicochemical properties, and regulatory hurdles. AustinPx addresses these challenges through early, thorough molecular characterization and a risk-mitigated, data-driven development strategy. They emphasize that skipping pre-formulation analysis to accelerate timelines can result in costly delays later.
Key insights:
- Early Risk Identification: Identifying issues like bioavailability, stability, and processability early avoids downstream development failures.
- Simplicity Through Strategy: While sponsors desire simple dosage forms, molecular complexity often requires advanced formulation strategies.
- Expert Partner Role: AustinPx uses its background as a sponsor to guide clients with deep scientific expertise and real-world experience.
Special Capabilities:
- HPAPI Handling: AustinPx has purpose-built infrastructure and in-house expertise for safely managing highly potent compounds (OEB 5).
- Bioavailability Enhancement (BAE): Their core strength is KinetiSol™, a leading amorphous solid dispersion (ASD) technology, enabling formulation of poorly soluble molecules.
- Innovative Testing & Tools: The team employs unique in-house tests, in vitro/in silico modeling, and rapid API-sparing screening for optimized development.
- Added Value:
AustinPx brings sponsor-level insight, allowing for agile, science-driven development with the goal of faster clinical entry and long-term product success.
KinetiSol™ stands out for its broad applicability, offering IP advantages and flexibility beyond conventional ASD methods.

Comments
No comments posted yet.