Scientific papers
Objectives: Children in need of cortisol replacement therapy often receive prescriptions for 2.5 mg hydrocortisone doses, which are not commercially available. Consequently, 10 mg tablets, equipped with functional break lines, are commonly split to achieve the desired dosage. This study aimed to assess the variation in dosage resulting from quartering hydrocortisone tablets when performed by different operators. Additionally, the study aimed to explore the potential for better uniformity through the use of mini-tablets as an alternative formulation.
Methods: Hydrocortisone 10 mg tablets were quartered by four different operators using a standard pill splitter. Hydrocortisone 2.5 mg mini-tablets (3 mm diameter) were formulated using a wet granulation method and produced using a high-speed rotary press simulator. The weight and content uniformity of both quartered tablets and mini-tablets were evaluated based on pharmacopoeial standards. The physical strength and dissolution profiles of the mini-tablets were also determined.
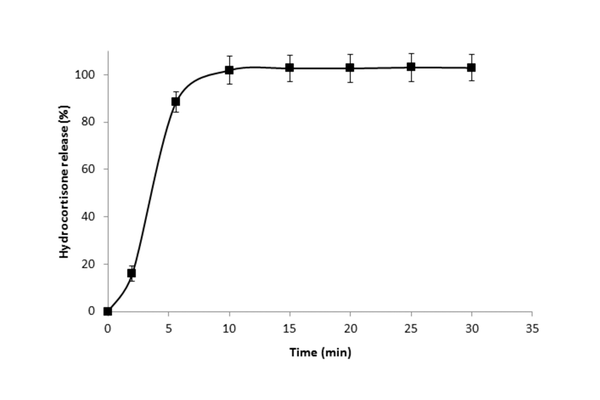
Results: Over half of the quartered 10 mg tablets deviated beyond ±10% of the stated US Pharmacopoeia hydrocortisone content (mean 2.34 mg, SD 0.36, coefficient of variation (CV) 15.18%). Additionally, more than 40% of quartered tablets fell outside the European Pharmacopoeia weight variation range. Robust mini-tablets (tensile strengths >4 MPa) were successfully produced, meeting pharmacopoeial weight and content uniformity requirements (mean 2.54 mg, SD 0.04, CV 1.72%) and drug release criteria during in vitro dissolution testing.
Conclusion: This study confirmed that quartering 10 mg hydrocortisone tablets leads to unacceptable dose variations. It also demonstrated the feasibility of producing 3 mm mini-tablets with more accurate doses for pediatric patients.
Comments
No comments posted yet.
Add a comment













