Scientific papers
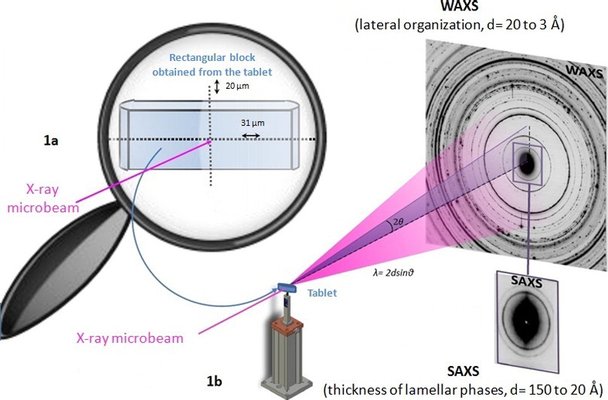
Lipid excipients are increasingly utilized in the pharmaceutical industry for sustained drug delivery, yet their development faces challenges due to the well-established polymorphism of lipids, often perceived as a drawback with potential implications for drug release during storage. To investigate any potential connection between drug release modification and lipid polymorphism, we employed synchrotron radiation-based micro X-ray diffraction. This technique allowed us to examine the crystalline structures of the lipid matrix-forming excipient at a local scale, scanning it throughout the entire tablet. The findings revealed that each tablet contains only one polymorph of Compritol® 888 ATO, and this polymorph remains consistent regardless of the applied compression force during manufacturing or after storage at 40°C for 45 days, despite variations in drug release profiles. Therefore, the observed modification in drug release after storage is not attributed to lipid polymorphism. Post-compression thermal treatments can generate another lipid polymorph, yet drug release remains unrelated to polymorphism, as two distinct polymorphs of Compritol® 888 ATO yield identical dissolution profiles. The fluctuations in drug release observed during storage in accelerated conditions may be attributed to an altered distribution of the lipid component within the matrix structure, where the lipid may flow and enhance the tablets' hydrophobicity.
Comments
No comments posted yet.
Add a comment