Scientific papers
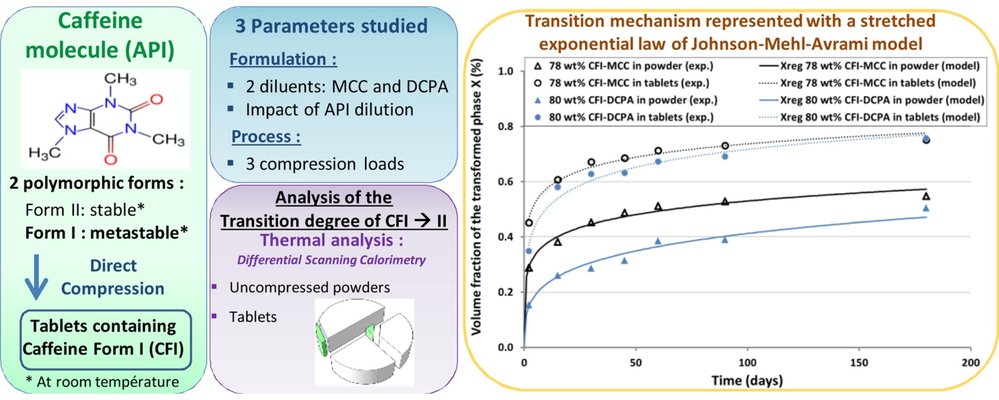
In the pharmaceutical sector, understanding solid-state transitions during the manufacturing of pharmaceuticals is crucial. This study focuses on analyzing the phase transition of a model API, caffeine Form I (CFI), during the direct compression process, considering the impacts of various operating conditions (process and formulation). Specifically, the investigation centers on two formulation parameters: the nature of the diluent and the influence of caffeine dilution, along with one process parameter: compression pressure. Tablets were produced using pure CFI and binary mixtures of CFI with diluents (microcrystalline cellulose or anhydrous dicalcium phosphate). A kinetic study conducted over six months revealed the effects of these parameters on the degree of CFI transition. The results indicated that the direct compression process had a triggering effect, leading to a higher transformation in tablets compared to uncompressed powders. Regardless of the applied pressure, the CFI transition degree remained relatively constant, uniformly occurring throughout the tablet volume. However, variations in the evolution of the CFI transition degree were observed among different binary mixtures of CFI and diluent. Analyzing the transition mechanism with a stretched exponential law of the Johnson-Mehl-Avrami model demonstrated that tableting accelerates the polymorphic transition without altering its nucleation-controlled mechanism.
Comments
No comments posted yet.
Add a comment














