Scientific papers
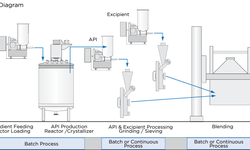
In the realm of pharmaceutical manufacturing, there is a growing interest in continuous manufacturing. This study focuses on enhancing the comprehension of twin-screw wet granulation as an example of a fast, complex continuous process. The investigation delves into the impact of the liquid-to-solid (L/S) mass flow ratio on both product quality (granules) and downstream process operations (tableting). The study initially employs two methods to establish L/S ratio boundaries for the granulation regime in twin-screw wet granulation. The first method, based on measuring the variation in wet granule mass flow, is utilized to define the upper L/S ratio boundary of the granulation regime. Meanwhile, the second method, centered on measuring granule size distribution, is applied to determine the lower L/S ratio boundary of the regime. Employing these methods facilitates the establishment of the granulation regime for different formulations. This information is then utilized to optimize the formulation for enhanced robustness, characterized by wider L/S ratio boundaries for the granulation regime. Additionally, it aids in optimizing the formulation with consideration for downstream processes like drying (minimizing water usage to reduce drying efforts) or tableting (producing granules with optimized tableting properties). Ideally, the process should be executed close to the lower L/S ratio boundary of the granulation regime. In summary, these tools enable the quantitative determination of granulation regime boundaries in a twin-screw wet granulation process, offering a means to optimize formulation and establish a robust process. Analogies to other continuous processes in entirely different applications can be envisioned.
Comments
No comments posted yet.
Add a comment