Scientific papers
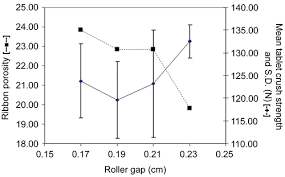
This paper discusses the utilization of an in-gap ribbon porosity calculation to optimize roller compaction ribbon parameters, aiming to regulate downstream granule and tablet properties in a typical pharmaceutical formulation. The study illustrates how alterations to roll speed and roll gap impact the relative degree of ribbon compaction for ribbons possessing comparable in-gap porosities. The findings highlight the applicability of in-gap ribbon porosity in facilitating the optimization of downstream granule processability characteristics for a typical pharmaceutical formulation. This approach provides valuable insights into the control space of a roller compaction process.
Comments
No comments posted yet.
Add a comment