Scientific papers
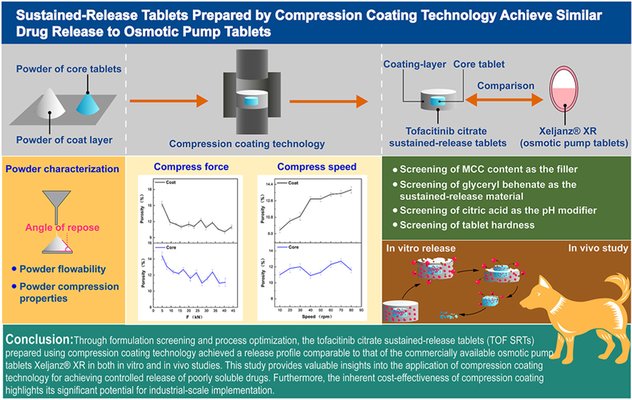
Over the past decades, various controlled-release technologies have been developed to achieve consistent drug release rates in vivo. Among these, osmotic pumps are widely used in commercial formulations due to their precise release control. However, their manufacturing typically involves additional steps—such as applying a semipermeable membrane and laser drilling—that add complexity compared to simpler techniques like compression coating.
This study explores compression coating (CC) as an alternative method to develop sustained-release tablets of tofacitinib citrate (TOF SRTs). By optimizing both formulation and processing parameters, suitable levels of glyceryl behenate were identified as the release-retardant (15% in the core and 30% in the coating), along with citric acid as a pH modifier (3% in both layers). The core tablet hardness was set at 25–30 N, and the final tablet hardness maintained between 100–110 N.
Under these optimized conditions, the resulting TOF SRTs demonstrated in vitro and in vivo release profiles closely aligned with the commercial product Xeljanz® XR, which is manufactured using osmotic pump technology. In vitro dissolution testing yielded a similarity factor (f2) of 80.66, while pharmacokinetic studies in Beagle dogs showed a relative bioavailability of 89.9%.
The key innovation lies in the broad applicability of this approach. By modulating the ratio of sustained-release agents and internal pH modifiers, and fine-tuning core hardness, this strategy can be extended to other BCS Class II/IV drugs such as apixaban and ibrutinib. This offers a promising, scalable alternative for the industrial production of high-bioavailability sustained-release formulations.
Comments
No comments posted yet.
Add a comment