Advances in co-processed excipients: multifunctional platforms for diverse pharmaceutical formulations
Excipients used in pharmaceutical dosage forms are a crucial and unique ingredient, offering numerous advantages to the final formulations, making it as important as the API. Benefits include enhancing API absorption and bioavailability, improving organoleptic properties, and ensuring the tablet is fully formed. However, selecting the right excipients remains challenging due to factors such as the diverse attributes of excipients, incompatibility between API and excipient, moisture absorption capacity, surface acidity, crystal nature, and the production of inferior, hazardous excipients.
The significant toxicity of excipients can greatly impact the pharmacokinetic and pharmacodynamic properties of the API and its dosage form, often resulting from the use of multiple excipients that perform inconsistently. While the pharmaceutical industry can mitigate excipient-related issues by adopting and enforcing cGMP, this does not prevent the production process from being affected by lengthy, costly, and laborious regulatory procedures. Co-processed excipients offer multiple functionalities, providing formulation scientists with unique options when developing dosage forms.
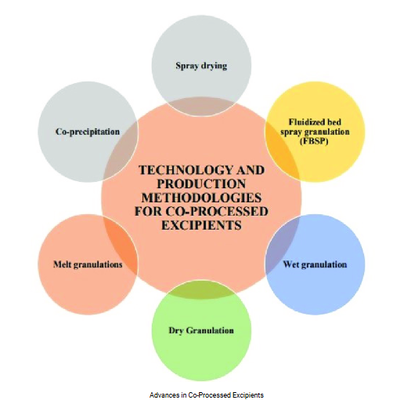
The need for co-processed excipients, their development techniques, risk and valuation studies, usage, and compliance with standards present an alternative and promising approach for selecting and utilizing an optimal combination of existing excipients over conventional ones in dosage form preparation.
Comments
No comments posted yet.