Amorphous solubility advantage: theoretical considerations, experimental methods, and contemporary relevance
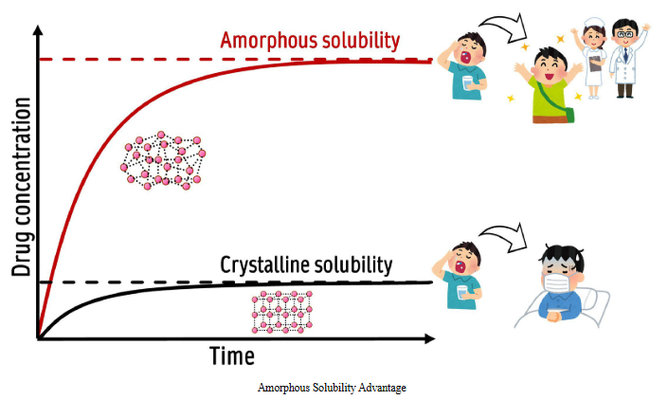
The concept of amorphous solubility has evolved significantly over the past 25 years, since Hancock and Parks posed the question of its true advantage for pharmaceuticals. Advances in theory and experimental techniques have clarified that amorphous solubility refers to the concentration of a drug after it undergoes phase separation, forming a water-saturated drug-rich phase in equilibrium with an aqueous phase containing dissolved molecules.
While crystalline solubility is crucial for absorption in crystalline drug formulations, amorphous solubility plays a key role in supersaturating formulations. However, measuring amorphous solubility is challenging due to complexities introduced by formulation additives and gastrointestinal factors, which can obscure the maximum thermodynamic activity of the drug.
This review examines the historical context, theoretical framework, and methods for measuring amorphous solubility, as well as its contribution to drug absorption. A deeper understanding of amorphous solubility and its associated principles can support the development of strategies for enhancing the absorption of poorly water-soluble drugs, improving therapeutic outcomes.
Comments
No comments posted yet.