Development of mini-tablet and multi-unit pellet systems (MUPs)
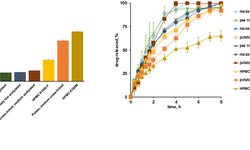
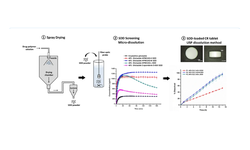
The text discusses the development and benefits of Mini-Tablet and Multi-Unit Pellet Systems (MUPS) in oral drug delivery. These advanced dosage forms offer controlled and sustained release profiles, achieved through various coatings and formulations.
MUPS and Mini-Tablet Systems (EMTS) provide advantages like reduced dose dumping, improved bioavailability, and enhanced efficacy with fewer side effects. They are suitable for various indications including gastrointestinal disorders, cardiovascular diseases, psychiatric conditions, pain management, and dysphagia. Additionally, MUPS and EMTS formulations address challenges posed by unstable or poorly soluble APIs, narrow therapeutic indices, and the need for specific release patterns. Excipients play a crucial role in these formulations, contributing to functionality and performance.
Challenges in development include ensuring stability, maintaining drug-excipient compatibility, and achieving uniformity in coatings and particle sizes. Advanced computational modeling techniques aid in formulation design and optimization, while comprehensive development strategies involve pre-formulation characterization, quality-by-design principles, and robust manufacturing processes. Patient-centric considerations include ease of swallowing, taste-masking, and improved palatability, contributing to enhanced patient adherence and treatment outcomes. The integration of formulation development, manufacturing, and clinical testing is key to reducing timelines and improving decision-making. Factors such as bioavailability, stability, and subject safety are major considerations in formulation.
The article provides case studies illustrating the challenges and solutions in pharmacy and manufacturing, emphasizing the need for collaboration and proactive integration between different aspects of drug development.
Comments
No comments posted yet.