Impact testing as a new approach to determine mechanical strength of pharmaceutical tablets
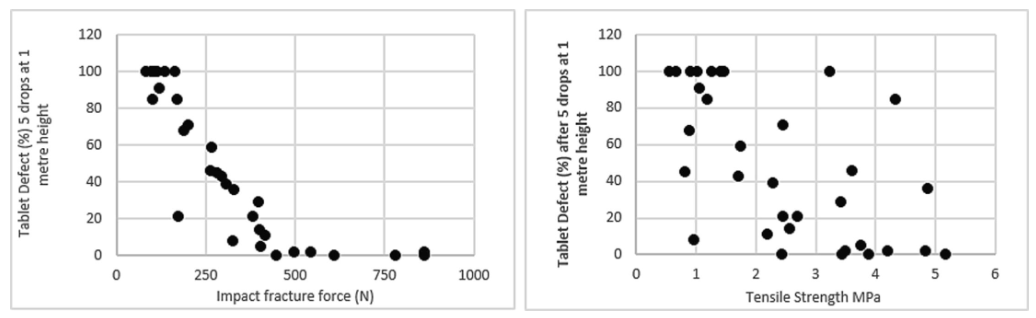
The paper discusses shortcomings in traditional standardized testing methods for determining tablet strength in the pharmaceutical industry, particularly focusing on the tablet breaking force test and friability test. These methods fail to accurately predict tablet defects during manufacturing, packaging, and shipping due to their inability to simulate rapid transfer of energy experienced by tablets. As an alternative, impact fracture force testing is proposed as a more effective method for assessing tablet mechanical strength. This method involves measuring the force absorbed by the material before fracturing under impact energy, providing superior correlation with tablet defect rates compared to traditional tests. Various studies and methodologies exploring alternative testing approaches are reviewed, highlighting the need for a test that can accurately predict tablet robustness during manufacturing while providing digital data with good repeatability. The study also introduces a drop-tower impact tester as a promising tool for simulating mechanical stresses experienced by pharmaceutical tablets and characterizing their strength.
Comments
No comments posted yet.