Improving API solubility using hot melt extrusion formulation with polyvinyl alcohol
The article from Pharmaceutical Online explores how hot melt extrusion (HME) using polyvinyl alcohol (PVA) can significantly improve the solubility and bioavailability of poorly water-soluble active pharmaceutical ingredients (APIs).
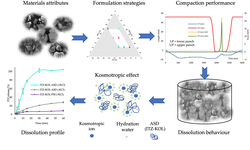
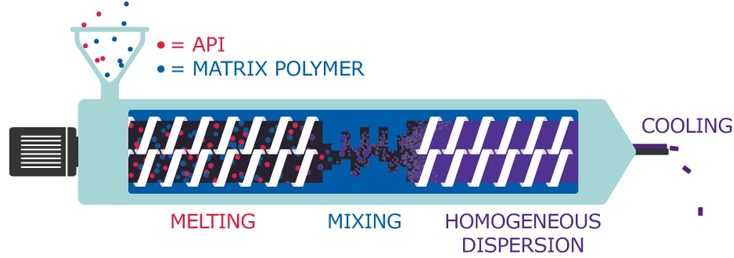
Poor solubility is a major challenge in drug development, often limiting the therapeutic efficacy of small-molecule drugs. HME addresses this by dispersing the API into a polymer matrix—in this case, PVA—creating a stable amorphous solid dispersion that dissolves more readily than its crystalline form. PVA is particularly effective due to its biocompatibility, film-forming properties, and ability to provide consistent drug release.
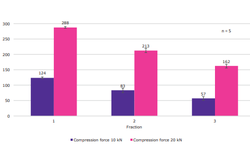
MilliporeSigma’s research highlights how different grades of Parteck® MXP PVA influence melt behavior and drug dissolution, showing how formulation choices can be tailored to optimize performance. HME is also a solvent-free, continuous, and scalable process, making it highly suitable for modern pharmaceutical manufacturing.
Overall, combining HME with PVA offers formulators a robust and efficient strategy to enhance the solubility and manufacturability of challenging APIs.
Comments
No comments posted yet.