Scientific papers
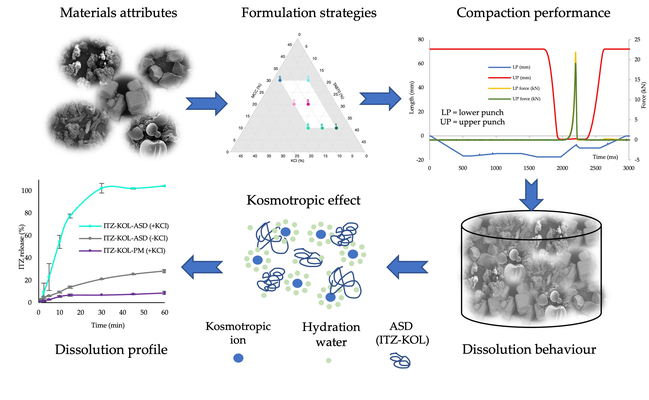
BCS Class II drugs, exemplified by itraconazole (ITZ), are characterized by poor solubility (1–4 ng/mL), necessitating solubility enhancement. Consequently, amorphous solid dispersions (ASDs) of ITZ and Kollidon® VA64 (KOL) were produced using hot-melt extrusion (HME) to address ITZ's limited solubility. A novel tablet formulation strategy involving five inorganic salts (KCl, NaCl, KBr, KHCO3, and KH2PO4) was explored. These kosmotropic salts are believed to compete for water hydration near the polymer chain, preventing polymer gelation and thereby facilitating disintegration and dissolution.
Among the formulations, the one containing KCl demonstrated acceptable tensile strength (above 1.7 MPa) and achieved rapid disintegration (less than 15 min). This formulation was chosen for further development using a design of experiment approach. Seven ITZ-KOL-ASD formulations with KCl were compacted using both round and oblong punches. Round tablets disintegrated in under 20 min, while oblong tablets disintegrated within 10 min. Round tablets achieved over 80% ITZ release within 15 min, with six out of seven formulations achieving 100% release by 30 min. It was observed that formulations with high levels of Avicel® pH 102 (30%) and low levels of KCl (5%) tended to fall short of the disintegration target due to the strong bonding capacity of Avicel® pH 102.
Disintegration time and tensile strength responses were modeled to define design spaces (DSs) applicable to both round and oblong tablets. Within the DS, multiple formulations meeting the Quality Target Product Profile (QTPP) requirements for immediate-release round and oblong tablets were identified, allowing flexibility to accommodate patients' needs with different tablet shapes. The study concluded that the use of inorganic salts, particularly KCl, is crucial for producing ITZ ASD tablets with fast disintegration and enhanced dissolution. Overall, the study successfully achieved ITZ-KOL-ASD tablet formulations meeting the QTPP, employing Quality by Design (QbD) principles for formulation and compaction process development and optimization.
Comments
No comments posted yet.
Add a comment













