Improving content uniformity in low-dose DC formulations
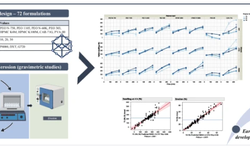
The case study focuses on the challenge of achieving content uniformity in tablets formulated using low-dose APIs via direct compression (DC). In such formulations, achieving uniform distribution of the active substance is difficult due to its low loading and potential segregation. The excipient composite PROSOLV® RX 90, developed by JRS Pharma, serves as an all-in-one “high functionality excipient” (HFE) for DC, integrating filler, binder, and lubricant into a single component.
Because PROSOLV® RX 90 is manufactured via a proprietary process that increases specific surface area and introduces micro-rugosity, it supports improved API distribution and flow when dealing with low dose APIs. The study with piroxicam tablets (8 mm, 100 mg and 200 mg doses) showed that using PROSOLV® RX 90 simplifies tableting, shortens development time and reduces complexity (fewer excipients to handle, less QC/storage overhead) when compared to conventional blends.
Key benefits highlighted include enhanced flow and compaction properties, less risk of segregation, and streamlined manufacturing. The authors stress that for low-dose DC tablets—where API loadings are small—selecting an excipient system designed for content uniformity and direct compression is crucial to ensure consistent tablet quality and manufacturability.

Comments
No comments posted yet.