Modified release formulations give new life to drugs
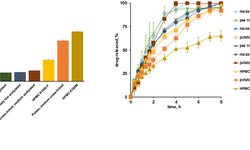
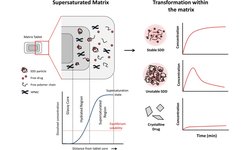
Modified‑release (MR) formulations — including **extended‑release (XR) tablets and controlled‑release solid dosage forms — are increasingly used not just for therapeutic benefit, but also as a lifecycle‑management strategy to extend product value as patents near expiration. This trend has been supported by regulatory pathways like the FDA’s 505(b)(2), which allows subtle changes in an approved drug’s dosage form (such as converting from immediate release to XR) to gain additional market exclusivity. Companies view MR tablet reformulations as a high‑impact lifecycle strategy that can offer patient benefits (such as reduced dosing frequency and improved compliance) while also providing economic value, despite being time‑ and resource‑intensive to develop and manufacture. Several contract development and manufacturing organizations (CDMOs) offer advanced technologies and expertise in MR formulation design, particle coating, and controlled‑release matrix development to support both clinical and commercial tablet products.

Comments
No comments posted yet.