Scientific papers
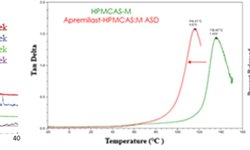
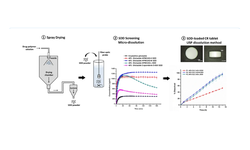
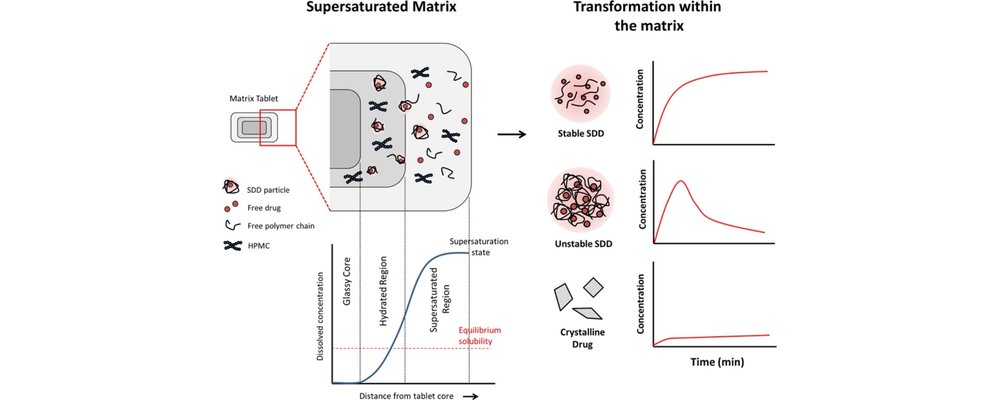
Spray-dried dispersions (SDDs) of glipizide, a model drug classified as BCS Class II, were created utilizing various hydroxypropyl methylcellulose acetate succinate (HPMCAS) grades and copovidone S-630 as carriers. Analysis through X-ray powder diffraction (XRPD), modulated differential scanning calorimetry (mDSC), and scanning electron microscopy (SEM) revealed that the SDDs exhibited a single amorphous phase, achieving drug loading levels of up to 60%. Supersaturated micro-dissolution testing conducted in fasted state simulated intestinal fluid demonstrated a prolonged supersaturation state, lasting up to 180 minutes, with solubility increases ranging from 5.2 to 13.9-fold compared to the crystalline drug under analogous conditions. The solubility and stability characteristics of the most desirable SDDs, as determined by relative dissolution AUCs (AUC(SDD)/AUC(crystalline)) and supersaturated concentration ratios (C180/Cmax), were investigated. Results indicated that HPMCAS-based SDDs achieved a higher degree of supersaturation compared to Copovidone S-630, and SDDs incorporating HPMCAS-M and HPMCAS-H maintained stable supersaturated concentrations. Dissolution data further demonstrated that controlled-release tablets loaded with SDDs maintained a stable supersaturated concentration within the hydrated matrix, exhibiting an increased rate and extent of drug dissolution over a 24-hour period. The coexistence of HPMCAS and HPMC within the hydrating matrix effectively suppressed drug crystallization, allowing for the attainment of zero-order and slow-first order release kinetics.
Comments
No comments posted yet.
Add a comment