Formulation considerations for pediatric populations: finding the right dosage form for better adherence
The text discusses the challenges and strategies for developing pediatric drug formulations to improve adherence and outcomes. It highlights the need for specialized technology platforms and expert partners to address the diverse preferences and physiological differences in children.
Key points include:
*Pediatric Formulation Challenges: Pediatric patients have diverse preferences and physiological characteristics that complicate drug formulation. Strict adherence to medication is crucial for efficacy and safety, but factors like taste, texture, and ease of swallowing can affect compliance.
*Factors Influencing Adherence: Eating and swallowing behaviors differ between children and adults. Younger children have unique anatomical features that affect how they swallow, necessitating formulations that are palatable and easy to ingest.
*Taste Masking and Texture: Mild flavors and smooth textures are universally preferred by children. Taste-masking techniques are essential for making bitter active pharmaceutical ingredients (APIs) more acceptable. Drug developers should consider these preferences to enhance adherence.
*Avoiding Off-Label Administration: Proper pediatric formulations reduce the need for caregivers to crush or mix medications with food, which can alter drug efficacy and safety. Adherence to correct formulations ensures consistent drug release and absorption.
*Partnering with Experts: Collaborating with experienced contract development and manufacturing organizations (CDMOs) like Adare Pharma Solutions can expedite the development of acceptable pediatric formulations. Adare's technologies, such as Microcaps® and Optimµm®, offer taste-masking, controlled release, and patient-friendly dosage forms like the Parvulet®.
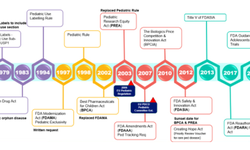
*Regulatory and Commercial Considerations: Regulatory bodies emphasize developing pediatric formulations early in the drug development process. Partnering with CDMOs helps meet regulatory standards and improves commercial success by ensuring formulations are tailored to pediatric needs.
The text underscores the importance of targeted formulation strategies and expert partnerships in achieving better patient outcomes and adherence in pediatric populations.

Comments
No comments posted yet.