In-line porosity and hardness monitoring of tablets by means of optical coherence tomography
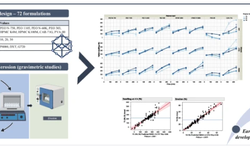
In-line monitoring of critical quality attributes (CQAs) during the tableting process is a crucial step toward implementing a real-time release strategy. These CQAs include tablet mass, API content, dissolution, hardness, and tensile strength. While dissolution testing is labor-intensive and time-consuming—and cannot be performed in-line—it is ideal to replace this testing with predictive models that focus on other CQAs influencing dissolution, such as tablet porosity and hardness. Traditionally, porosity has been measured offline using techniques like gas adsorption, Terahertz spectroscopy, or gas-in-scattering media absorption spectroscopy.
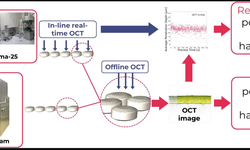
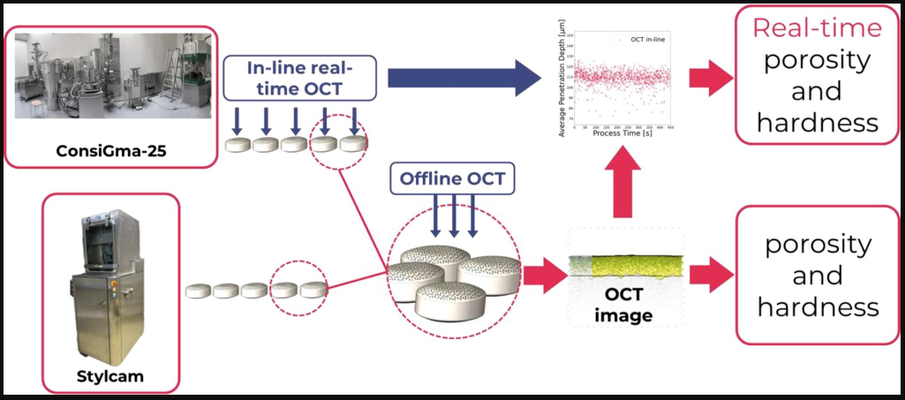
Tablet hardness is typically evaluated using a hardness tester. Though these destructive tests are easily performed at-line, they have limited use in high-percentage in-line inspections. Recently, Optical Coherence Tomography (OCT) has been proposed as a promising tool for evaluating quality attributes. This study introduces the first application of OCT to predict tablet porosity and hardness. OCT measurements were conducted on tablets produced using the ConsiGma 25™ tableting line and the Stylcam 200R compaction simulator at various compaction force settings, with the results correlated to porosity and hardness.
The study demonstrated that OCT can be seamlessly integrated in-line, providing real-time data on critical material attributes. This real-time insight supports the viability of OCT as a quality control tool, with the potential to replace time-consuming and destructive offline measurements.
Two types of tablets were used in this study. One set was produced using five different compaction forces in the continuous tableting line, while another set was made at six compaction forces in the Stylcam 200R compaction simulator. The OCT system, OSeeT Pharma 1D (Phyllon GmbH, Austria), was installed directly after the tablet press above a conveyor belt during the tableting process.
Access the "Papers" section for more information.
Comments
No comments posted yet.