How To achieve different release profiles and select excipients for formulation development of modified release oral solid dosage forms
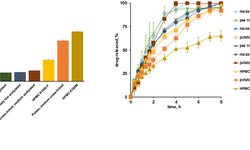
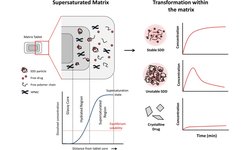
Modified release oral solid dosage forms (MROSD) are specialized formulations that enable drug release over a defined period or at specific GI tract locations, improving patient adherence and reducing side effects. Achieving the desired release profile requires a comprehensive approach, considering the API's properties, appropriate excipients, and manufacturing techniques. Key factors include understanding the pharmacokinetics, site of absorption, drug solubility, powder properties, and particle size distribution.
High drug loads and multiple APIs add complexity, requiring careful selection of delivery systems (matrix tablets, capsules, multiparticulates) and excipients. Release-controlling polymers and other excipients are chosen based on their impact on release rates and processability. A thorough understanding of these elements ensures consistent, effective dosage forms.
Partnering with experienced manufacturers, like Societal, can help navigate these complexities, leveraging decades of expertise in modified release formulations to achieve desired therapeutic outcomes.

Comments
No comments posted yet.