Winning strategies for oral dosage form development and manufacturing
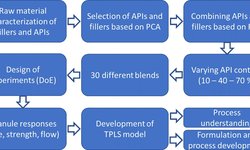
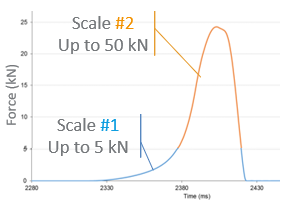
This article outlines practical strategies for oral solid-dose (OSD) formulation development, emphasizing the importance of front‑loaded risk assessment and molecular characterization to preempt failure due to poor bioavailability, stability, or processability. It advocates building transparent collaboration between sponsors and CDMOs, ensuring clear timelines and regulatory alignment. The author underscores that comprehensive pre-formulation data—including physicochemical, stability, and manufacturability profiles—serves as a critical foundation that de‑risks development and speeds clinical translation. By identifying key red flags early, development teams can streamline decision-making and increase the likelihood of success in clinical and commercial stages.

Comments
No comments posted yet.