Scientific papers
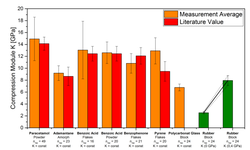
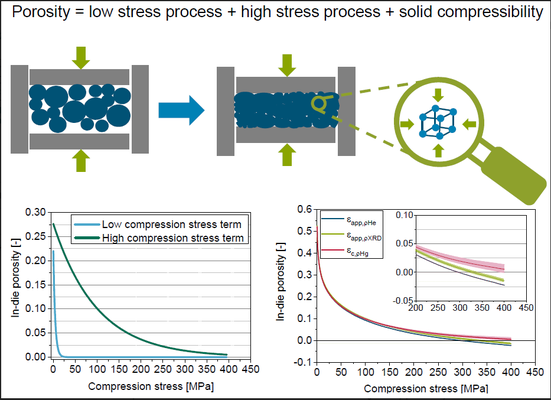
The analysis of in-die compression proves to be a valuable method for powder compressibility characterization. However, issues arise when calculating physically implausible apparent solid fractions exceeding one or apparent in-die porosities falling below zero, especially under higher compression stresses. This often stems from overlooking solid compressibility and assuming a constant solid density. In this study, we characterize the solid compressibility of four pharmaceutical powders with distinct deformation behaviors using mercury porosimetry. The derived bulk moduli are then utilized to compute in-die porosities. Considering solid compressibility leads to a notable change in in-die porosity, reaching up to 4% for microcrystalline cellulose at a compression stress of 400 MPa, emphasizing its significance in the calculation. However, solid compressibility, coupled with uncertainties from factors like measured solid density and displacement sensor accuracy, poses challenges in accessibility or understanding. To address this, a mathematical term is introduced to calculate physically reasonable in-die porosities. This term can enhance existing mathematical models, including Heckel and Cooper & Eaton models. Additionally, an extended in-die compression function is proposed to precisely characterize the entire range of in-die porosity curves, facilitating a nuanced understanding and quantification of the compression behavior exhibited by the investigated pharmaceutical powders.
Comments
No comments posted yet.
Add a comment