Scientific papers
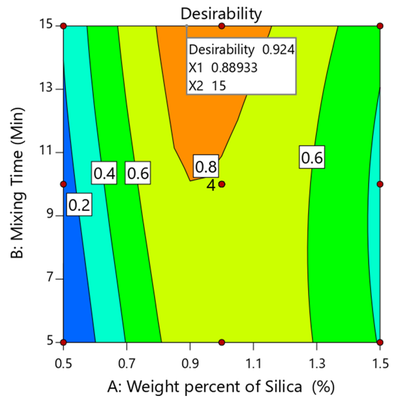
Creating a robust direct compression (DC) formulation for an active pharmaceutical ingredient (API) with challenging flow and compaction properties at a high API load poses a considerable challenge. This study addressed two key issues: the adverse flow characteristics and tableting challenges associated with a high-drug-loading canagliflozin (CNG), enabling high-speed DC tableting. This was achieved through a one-step dry coating process utilizing hydrophilic nano-sized colloidal silica. A 32 full-factorial experimental design was implemented to optimize the independent process variables, namely, the weight percent of silica nanoparticles (X1) and mixing time (X2). The study delved into the flow, bulk density, and compaction properties of CNG–silica blends, and the optimized blend was subsequently compressed into tablets using the DC technique. Regression analysis revealed a significant (p ≤ 0.05) influence of both X1 and X2 on the characteristics of CNG, with X1 having a predominant effect. Moreover, tablets produced from the processed powders demonstrated robustness compared to those from the control batch. Additionally, these tablets exhibited significantly lower tablet ejection forces than those from the control batch, highlighting the lubrication impact of the silica nanoparticles. Notably, these tablets showcased improved disintegration time and dissolution rates. In summary, a dry coating process utilizing silica nanoparticles provides an opportunity to address the poor flow and tableting challenges of CNG, while minimizing the need for excessive excipients. This is crucial for the effective development of a small-sized tablet and achieving a cost-effective manufacturing process.
Comments
No comments posted yet.
Add a comment