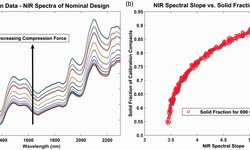
Scientific papers
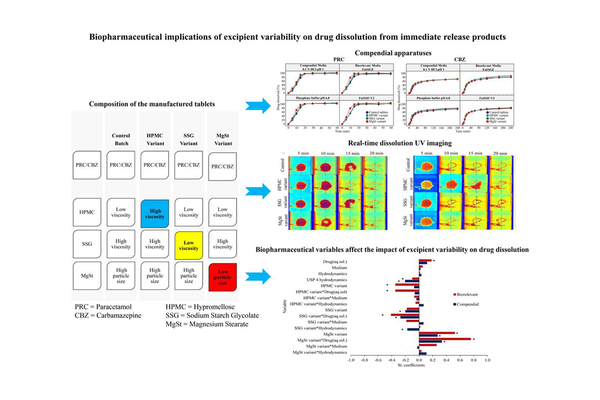
Examining the influence of excipient variability on oral product performance from a biopharmaceutical perspective could be advantageous and facilitate the integration of excipients into Quality by Design (QbD) approaches. The present study explored the effects of varying viscosity in binders (hypromellose (HPMC)) and superdisintegrants (sodium starch glycolate (SSG)), as well as the particle size distribution of lubricants (magnesium stearate (MgSt)), on the in vitro dissolution of both highly and poorly soluble drugs in immediate release formulations.
Compendial media, including pharmacopoeia buffers, and biorelevant media simulating gastrointestinal fluids were employed, along with the USP 2 and USP 4 apparatuses, to assess the impacts of the varied excipients on drug dissolution. Real-time dissolution UV imaging provided mechanistic insights into the disintegration and dissolution processes of the immediate release formulations. Altering the viscosity type of HPMC or SSG did not significantly affect drug dissolution, regardless of the compound used. Faster drug dissolution was observed by reducing the particle size of MgSt for the highly soluble drug.
Real-time dissolution UV imaging highlighted the influential role of excipient variability in tablet disintegration. For the highly soluble drug, tablets containing high viscosity HPMC or low particle size MgSt disintegrated faster compared to control tablets. Conversely, for the poorly soluble drug, slower tablet disintegration occurred when increasing the viscosity of the HPMC compared to the control tablets. Changes in drug dissolution due to varying excipients may be expected if the excipient change has previously impacted drug solubility. Multivariate data analysis revealed critical biopharmaceutical factors, including specific excipient types/properties, drug aqueous solubility, and medium/hydrodynamic characteristics, affecting the impact of excipient variability on in vitro drug dissolution.
Comments
No comments posted yet.
Add a comment