Scientific papers
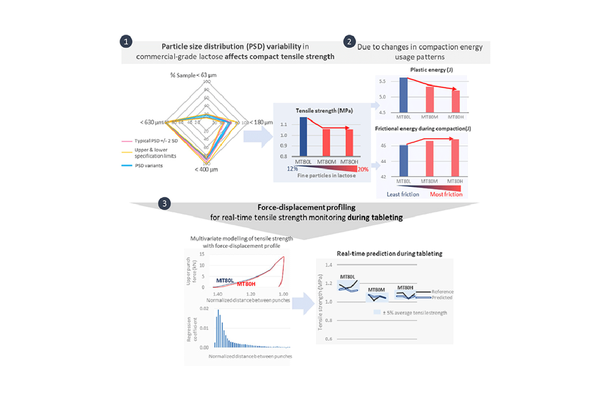
Background: The sensitivity of direct compression to the variability in particle size distribution (PSD) of pharmaceutical-grade excipients remains insufficiently explored. Additionally, the utilization of a force sensor as a process analytical technology (PAT) platform for monitoring the impact of PSD variations on compact tensile strength is a readily available but underutilized strategy.
Methods: To address these gaps, we investigated the effect of PSD variability on compaction. We tableted low (4% w/w drug) and high (15% w/w drug) dose blends containing chlorpheniramine, microcrystalline cellulose, and spray-agglomerated lactose. The PSD of spray-agglomerated lactose was manipulated by introducing ungranulated fines to simulate commercially relevant variability. Tensile strength and disintegration time were measured. The use of a force sensor to generate force-displacement and force-time profiles for in-line tensile strength prediction was evaluated.
Results: An increasing proportion of ungranulated fines (≥ 16%) resulted in a 10% and 4% reduction in tensile strength for low and high dose formulations (p < 0.02). The heightened friction during compaction impeded particle movement and diminished the energy available for bonding. However, disintegration performances remained equally acceptable for immediate drug release (≈ 30 s). Modeling tensile strength with force-displacement and force-time profiles achieved ≥ 98% accuracy for in-line prediction (relative root mean square error of prediction = 3.7% and 4.8%).
Conclusion: This study enhances our understanding of the relationship between PSD variability and direct compression. The use of force-displacement and force-time profiling emerges as a practical and sophisticated PAT strategy. Notably, with further enhancements, the force sensor in the rotary tablet press can be repurposed for process monitoring and quality inspection. This opens up opportunities for improved process understanding and control without requiring additional investments in PAT platforms.
Comments
No comments posted yet.
Add a comment