Scientific papers
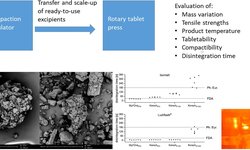
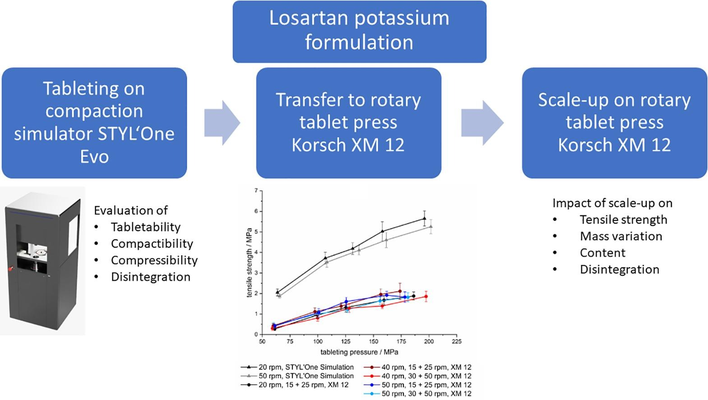
Mini-tablets (MTs) containing losartan potassium were developed for the treatment of the rare disease Epidermolysis Bullosa. The primary focus was on the transfer and scale-up of a direct compressible formulation from the compaction simulator STYL’One Evo (CS) to the rotary tablet press Korsch XM 12 (RP). The transfer of tabletability and compactibility profiles from CS to RP did not exhibit close agreement; for instance, at a tableting pressure of 125 MPa, mean tensile strengths (TS) were 4 MPa on CS and 1-1.5 MPa on RP. These results underscore the significant impact of the feed frame on final product qualities, influenced by various process and material factors.
In the scale-up studies, critical quality attributes (CQAs) such as mass variation, content uniformity, TS, and disintegration time were thoroughly examined. After an appropriate run-up time, most CQAs reached a plateau, establishing a balance between the influx, efflux, and distribution of lubricant in the feed frame. TS values ranged from 1-2 MPa, disintegration times peaked at 50 s, mass variation exhibited a range of 0.9-2.2% (CV), and acceptance values remained below 15.0. The specific outcomes were contingent upon the chosen process parameters.
Comments
No comments posted yet.
Add a comment