Scientific papers
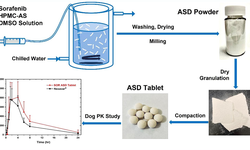
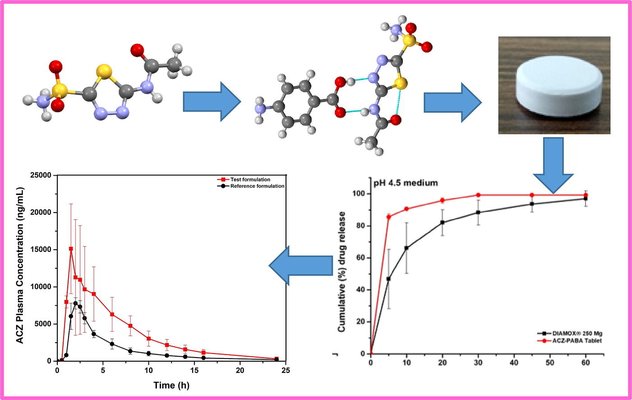
Pharmaceutical cocrystallization is widely applied to enhance the physicochemical attributes of Active Pharmaceutical Ingredients (APIs). However, the formulation of cocrystals with demonstrated clinical success is currently limited. The successful translation of a cocrystal into appropriate dosage forms necessitates the simultaneous improvement of multiple deficient physicochemical properties compared to the parent API, without compromising other crucial properties essential for successful product development. In this study, we detail the successful creation of a direct compression tablet product containing acetazolamide (ACZ), utilizing a 1:1 cocrystal of acetazolamide with p-aminobenzoic acid (ACZ-PABA). The ACZ-PABA tablet demonstrates superior biopharmaceutical performance in comparison to the commercial tablet DIAMOX® (250 mg) in healthy human volunteers, resulting in a more than 50% reduction in the required dose.
Comments
No comments posted yet.
Add a comment