Scientific papers
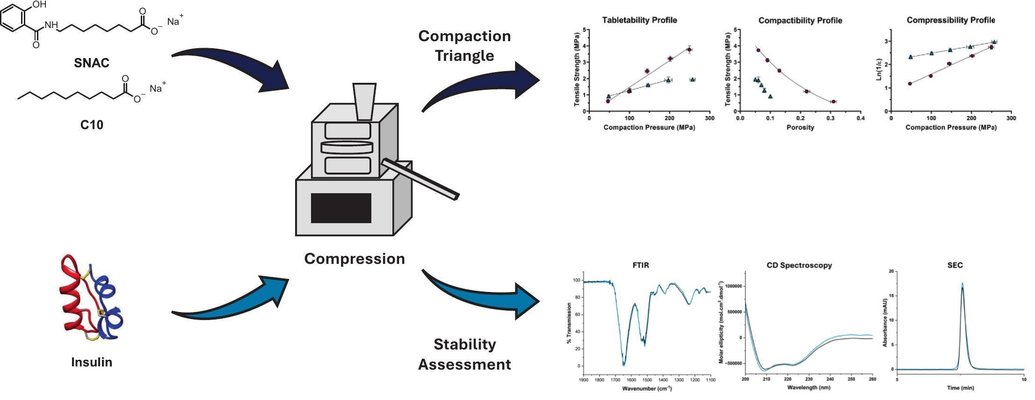
Recent breakthroughs in oral peptide delivery have largely stemmed from the use of chemical permeation enhancers such as sodium caprate (C10) and salcaprozate sodium (SNAC). However, combining peptides with these enhancers in oral dosage forms presents formulation and processing challenges that require deeper understanding. This study aimed to streamline the development of direct compression insulin compacts using C10 and SNAC as model enhancers. Researchers first examined the physical and mechanical characteristics of both materials to determine their suitability for direct compression. While C10 showed acceptable flowability, it exhibited poor tabletability, compactibility, and compressibility. In contrast, SNAC demonstrated better compaction behavior but lacked good flow properties. By incorporating microcrystalline cellulose (MCC) and polyvinylpyrrolidone (PVP) K30, the compaction performance of both materials improved. Optimized formulations containing 72% C10 or SNAC, 20% MCC, 5% PVP, and 3% insulin were successfully compressed at 100 and 200 MPa, producing tablets with complete insulin release within 30 minutes, unaffected by compaction pressure. Furthermore, insulin remained stable under mechanical stress and after one month of storage at 40 °C/75% RH, with reduced deamidation and aggregation compared to raw insulin. These findings highlight how formulation components influence the manufacturability, stability, and performance of peptide tablets produced by direct compression.
Comments
No comments posted yet.
Add a comment