Scientific papers
Metformin hydrochloride (MF) is a drug used in the treatment of type 2 diabetes. It is administered orally in the form of tablets with a high drug load. Due to the poor flowability and high agglomeration tendency of the material, powder blends cannot be
directly compressed into tablets. Milling followed by an intermediary production step, such as dry granulation, is necessary for tablet production.
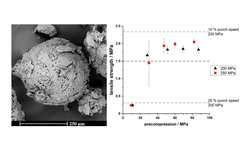
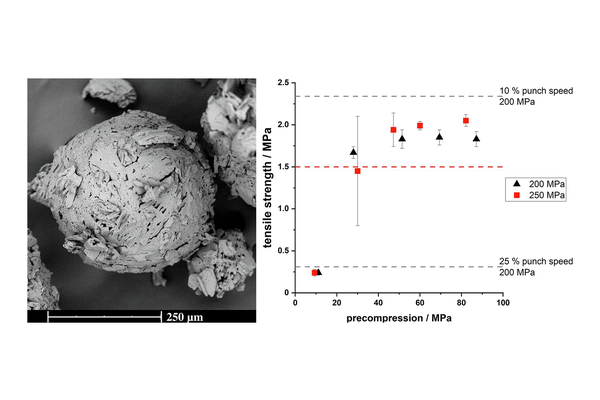
Quasi-emulsion solvent-diffusion (QESD) crystallization is a type of spherical crystallization, alongside spherical agglomeration, which can be used to improve the flowability and tabletability of an active pharmaceutical ingredient or excipient.
Hereby, crystallization and agglomeration occur in a single step inside transient emulsion droplets. An antisolvent crystallization is performed where the rate of counter-diffusion of the solvents is reduced by the addition of surfactants. The API solution within the droplets becomes supersaturated so that a spherical crust of the material is formed where further solute can crystallize.
In a previous study, a QESD crystallization technique was developed for MF. An aqueous solution of MF containing HPMC was crystallized in acetone. Polysorbate 80 and Span 80 were added to further stabilize the transient emulsion. A free-flowing
powder with reduced agglomeration tendencies under storage could be produced.
The aim of this study was to evaluate whether QESD MF can be directly compressed into tablets with a high drug-load (> 89.5 %) and if the surfactants used to stabilize the crystallization emulsion influence the strength of tablets.
Comments
No comments posted yet.
Add a comment