Scientific papers
Metformin hydrochloride, employed in treating type 2 diabetes, exhibits notable challenges with poor flowability and agglomeration during storage, making direct compression into tablets a hitherto unsuccessful endeavor. A previous study demonstrated that a quasi-emulsion solvent-diffusion (QESD) crystallization technique can significantly enhance flowability and diminish storage agglomeration of this drug. This current study aimed to assess the feasibility of directly compressing QESD metformin hydrochloride into high-dose tablets (> 89.5% drug load) without an intermediary step like granulation.
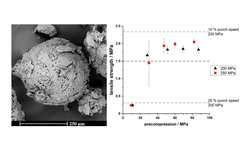
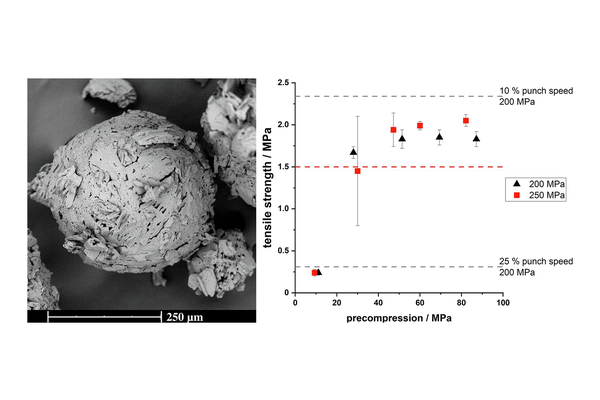
Successful direct compression into tablets was achieved; however, it was crucial to evaluate the tabletability of the material under the actual production speeds of the tablet press. The porous structure of the metformin agglomerates posed challenges related to deaeration, which could be mitigated by reducing punch speed or incorporating a precompression step. Additionally, the impact of surfactants used to stabilize QESD crystallization on the strength of produced tablets was analyzed, as the literature is still limited in coverage on this topic.
Comments
No comments posted yet.
Add a comment